by
John R. Fischer, Senior Reporter | September 19, 2023
Medical imaging AI software developer Annalise.ai has become the first company to score FDA breakthrough status for a radiology triage device.
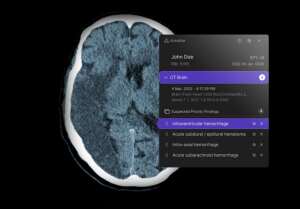
The Breakthrough Devices Program, which identifies solutions that improve the treatment or diagnosis of life-threatening conditions, will allow providers to quickly acquire the company’s obstructive hydrocephalus software tool, which provides passive and active notifications for suspected OHCP cases identified on unenhanced head CT scans. The application also received 510(k) clearance.
In cases of obstructive hydrocephalus, cerebrospinal fluid accumulates in the cranial vault, increasing intracranial pressure that can grow rapidly if not treated, risking permanent brain damage and death. Using deep-learning technology, Annalise.ai’s application identifies signs of this condition and elevates these cases in workloads so they can be prioritized immediately.



Ad Statistics
Times Displayed: 174434
Times Visited: 3180 For those who need to move fast and expand clinical capabilities -- and would love new equipment -- the uCT 550 Advance offers a new fully configured 80-slice CT in up to 2 weeks with routine maintenance and parts and Software Upgrades for Life™ included.
“With its set of clearances, Annalise.ai promotes faster report turnaround times by identifying and elevating critical cases for immediate attention,” said Dr. Rick Abramson, chief medical officer at Annalise.ai, in a statement.
The algorithm is one of the ten in Annalise.ai’s portfolio for U.S. hospitals that have been cleared by the FDA, five of which are for chest X-rays and five for head CT scans. The OHCP software tool is part of its Annalise Triage CTB platform.
Earlier this year, the company announced that it
received FDA 510(k) clearances for seven additional findings as part of its AI-assisted triage and notification (CADt) solutions. This included time-sensitive stroke conditions and chest X-ray findings.
The company has also expanded its global footprint recently, announcing in May that it would be
opening the Annalise India Centre in Chennai, its first establishment in India, to aid product development and marketing in Asia.