by
John R. Fischer, Senior Reporter | January 21, 2019
MIM Software has been given the go-ahead by the FDA to equip providers across the U.S. with its MIM SurePlan MRT software for molecular radiotherapy dosimetry.
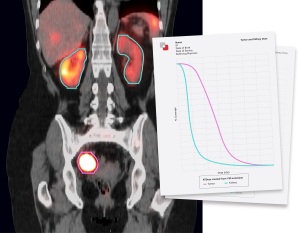
The clearance of the solution will enable clinicians to measure the absorbed dose from MRT for individual patients, a task that many were not able to perform due to the lack of available SPECT images and tools.
“Until recently, you've had to purchase a new SPECT/CT camera to do it," Aaron Nelson, M.D., chief medical officer of MIM Software, told HCB News. "Now, you can do it with your existing cameras.”



Ad Statistics
Times Displayed: 364924
Times Visited: 6942 Quality remanufactured Certified Centrifuges at Great prices! Fully warranted and backed by a company you can trust! Call or click for a free quote today! www.Centrifugestore.com 800-457-7576
Using a patient’s own images, MIM SurePlan MRT creates a quantitative SPECT reconstruction and voxel-based absorbed dose calculation for personalized dosimetry measurements. It also provides timesaving tools for organ and tumor segmentation, deformable registration, and voxel-based dosimetry for molecular radiotherapy.
Additional aid can be found in the software’s multi-tracer theranostics support, quantitative SPECT and planar corrections, and dosimetry reporting tools.
An effective form of therapy, MRT often relies on radiopharmaceuticals such as Lutathera and Azedra to target tumors based on certain receptors they express. Cleared in 2018 by the FDA, Lutathera has shown great success, as evidenced by its use in a first-in-human study that
was presented at the 2018 Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI).
MIM Software says the range of applications for MRT is expected to grow.
“Its most common uses include treatment for neuroendocrine (Lu-177 Dotatate), thyroid (I-131 NaI), and pheochromocytoma/paraganglioma (I-131 iobenguane),” said Nelson. “There are many others that are showing value, but aren't FDA-cleared, such as PSMA for metastatic castrate resistant prostate cancer.”
The software will be on display this month at the SNMMI 2019 Mid-Winter Meeting in Palm Springs, California.