by
Lauren Dubinsky, Senior Reporter | April 10, 2014
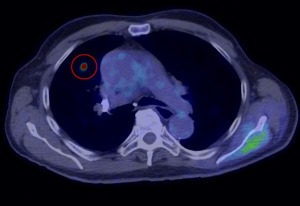
Courtesy of GE Healthcare
GE Healthcare announced that its new technology for improving quantitative accuracy and image quality in PET/CT imaging received U.S. Food and Drug Administration clearance.
According to GE, the new technology, called Q.Clear, will give clinicians full convergence PET imaging with both quantitation and image quality.
"We know that approximately 70% of cancer patients don't always respond to their initial course of treatment," Steve Gray, president and CEO of GE Healthcare MICT, said in a statement. "If we can give clinicians an accurate, reliable, and faster tool to confirm that a change in treatment is needed, the patient will benefit greatly."



Ad Statistics
Times Displayed: 172955
Times Visited: 3140 For those who need to move fast and expand clinical capabilities -- and would love new equipment -- the uCT 550 Advance offers a new fully configured 80-slice CT in up to 2 weeks with routine maintenance and parts and Software Upgrades for Life™ included.
Other than detecting smaller lesions, clinicians also need to find out if a patient is responding to treatment. When Q.Clear pairs with GE's Q.Suite, clinicians get better access to their patients' treatment response.
"Q.Clear is a major step forward because it can give us a consistent and reliable measurement when determining whether the current course of a patient's cancer treatment is effective," Dr. Gustav von Schulthess, Nuclear Medicine Chair at University Hospital Zurich, said in a statement. "It will give the oncologist more confidence because if a change of therapy is needed, you want accurate information early on, to best adjust treatment for the patient."

