Special report: Defibrillator data proving critical
July 16, 2012
by Loren Bonner, DOTmed News Online Editor
This article first appeared in the July 2012 issue of DOTmed Business News
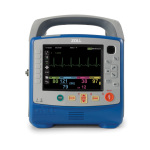
A few years ago, Redmond, Wash.’s local fire department received some discouraging news from the county medical director. They were told their service had a recorded CPR compression hands-on ratio of 66 percent—the lowest in the entire county. They knew about the data emphasizing increased compression time to increase resuscitation, so they quickly went to work on a performance improvement plan using new defibrillators they recently purchased from Physio-Control.
After resuscitation, Physio-Control’s LIFEPAK 15 defibrillator would transmit data via high-speed modem through Physio-Control’s LIFENET System to the CODE-STAT database at Redmond Medic One. The system would then send an alert to the smartphones of paramedics designated as “annotators” to let them know they had a case to review. “By the time [responders] got back to the station, they had instant feedback,” says Cam Pollock, global marketing vice president at Physio-Control.
After six months testing out this new feedback system, Redmond Medic One received more news from the county medical director. Their CPR compression hands-on-ratio was now the highest in the country at 83 percent.
“It improved over 20 points in six months, just by feeding back their performance on a quick basis,” says Pollock.
Improving patient outcomes through data
The American Heart Association updates its guidelines for CPR and ECC every five years. The most recent update, from 2010, highlights the need for good data.
“You can’t improve what you can’t measure. If you want to improve patient outcomes, you have to have a good way to collect and analyze,” says Pollock. The LIFENET system is cloud-based and connects to all of Physio-Control's products. Out in the field, not only would the device be able to feed back data to a local station to help paramedics improve their skills, but it could also send data to a local hospital in order for adequate preparations to be made for the patient’s arrival.
Recent AHA guidelines also urged high-quality compressions during CPR, specifically two inches of depth compression and at least 100 compressions per minute. But anyone who has ever performed CPR knows this is hard to maintain.
“Having a device deliver that instruction to the user for CPR is equally as important as the shock in my opinion,” says Ben Wellons, president of eMed Healthcare, a defibrillator distributor based in Little Rock, Ark.
CPR solutions
The top makers of defibrillators all incorporate several pieces of equipment and software that provide direct feedback to users to improve CPR numbers.
“CPR is challenging for people and unless you apply measurement and feedback, the user may not realize the quality,” says Jamie Froman, director of marketing for Philips Healthcare’s HeartStart automated external defibrillators.
Philips’ advanced life support HeartStart defibrillators come with quality CPR measurement and feedback. A little monitor that’s connected to the defibrillator sits on the patient’s chest. It helps measure the depth and rate of the compressions and gives the end user feedback on a display screen so that they can adjust their technique accordingly.
Zoll Medical Corporation, which was recently acquired by Japan’s Asahi Kasei Corp., provides Real CPR Help and real-time audio and visual feedback on all of its defibrillators to coach rescuers to the correct depth and rate of chest compressions as recommended by the AHA Guidelines. A few of Zoll’s defibrillators offer See-Thru CPR, which minimizes interruptions during CPR.
“The user is getting visual feedback on the important elements of quality and they are getting audible feedback when they are out of range,” says Ward Hamilton, senior vice president and vice president of marketing for Zoll. But Hamilton stresses the importance of the kind of feedback received.
“We tried to make sure it’s enough feedback but not too much that it interferes or you tune it out,” he says.
Data management is also a critical part of Philips’ advanced life support HeartStart line. “We can acquire a 12-lead ECG from a patient and send that to a receiving hospital in order for them to determine what hospital they should go to and the appropriate pathway of care,” says Froman. All the data on the device also helps guide the patient’s post treatment of care.
Zoll defibrillators with See-Thru CPR and Real CPR Help affected the University of California San Diego medical team. They tripled survival rates using the devices along with a “resuscitation bundle” as a new paradigm of care — again, using feedback to guide CPR performance and data analysis to aid in team training.
Taking this a step further, Physio-Control’s fastest growing product, the LUCAS 2 chest compression system, is a portable device that’s strapped onto a patient’s chest during cardiac arrest. It delivers automated, consistent chest compressions that aren’t compromised by a professional tiring out.
“Most people don’t do CPR often enough to become experts. In other words, quality declines quickly and rescuers often have to switch off, leaving gaps in the CPR flow. That affects not only overall survival, but also defibrillator efficacy. You want good CPR being done up until the shock. The longer you wait with hands off the chest, the defibrillator shock is less effective,” says Pollock.
Emergency kits
The Zoll defibrillators used by the UC San Diego team also had built in End-Tidal Carbon Dioxide monitoring to guide the quality of the CPR. Users can look for these values, displayed during cardiac arrest, to adequately gauge blood flow and O2 exchange during resuscitation.
Capnography—the measurement and numerical display of End-Tidal Carbon Dioxide—is another important feature highlighted in the 2010 AHA guidelines.
“So if you’re doing CPR and you have a low capnography, then you need to push harder. And there are some recommendations in the guidelines that say if you’re not at a certain level, you should be doing better CPR. It’s really becoming a measure of overall resuscitation,” says Pollack.
The LIFEPACK 15 has capnography measurements built in, but they also sell a standalone capnography module for people who want a hand-held device. Pollack describes this unit as a portable resuscitation unit or emergency care unit because it can do many more things than defibrillation, monitoring and pacing.
Some of the newest parameters on the device are temperature monitoring for hypothermia and a carboxyhemoglobin feature to detect carbon monoxide in the blood and methemoglobin in the blood, which results from exposure to certain drugs and chemicals.
Smaller but just as powerful
This year, manufacturers have released smaller and more compact defibrillators. Zoll’s lightweight, portable X Series Monitor/Defibrillator received 510(k) clearance from the Food and Drug Administration in March.
“We think it’s going to be a game-changing product and will set a whole new standard in that category,” says Hamilton. The device is being marketing to the pre-hospital environment and provides every advanced monitoring and communication capability required by EMS providers. But in a device that’s less than 12 pounds— nearly half the weight of most others on the market.
“Especially when you have to move equipment to a patient’s side, size and weight are key. Sometimes you can get small size, but you have to compromise the screen or features. Here, you have it all and you don’t have to give up anything to get the small size and the light weight,” says Hamilton.
He also points out that the device has two new capabilities: the ability for paramedics to communicate over wireless and to export data onto a USB port.
Phillips claims its new HeartStart FR3 is the smallest and lightest on the market for professional responders at less than four pounds. Some improvements in the device focus on workflow solutions for the users — all based on feedback from EMTs working with Philips’ devices out in the field. One improvement Froman points to is the pre-connected pads. “The engineers have found a way to take the bags off the pad so it removes a step from the workflow when time is of the essence,” he says. The device also has a bilingual capability that Froman thinks will give certain communities access to a wider range of responders.
“As we observe clinicians and ask for feedback to put into our products, over time, some of the features like Q-CPR that we have in the hospital and emergency medical space might migrate into other spaces. We fully expect that to happen,” he says.
Names in boldface are Premium Listings.
Domestic
Katherine MacCall, Soma Technology, CT
Leslie Roberts, Altra Medical, FL
Allyn Cutts, American Med Supply, FL
Moshe Alkalay, Hi Tech Int'l Group, FL
DOTmed Certified
Ronald Tarr, MEDELCO, FL
Todd McCuaig, LS Inc, IL
Alda Clemmey, Saffire Medical, MA
DOTmed Certified
DOTmed 100
John Gladstein, Medical Device Depot, MD
DOTmed Certified
Robert Schirano, Finger Lakes Medical Supply LLC, NY
Lawrence Maroney, Integris Equipment, NY
DOTmed Certified
International
Mads Vittrup, AGITO Medical, Denmark
DOTmed 100
Manohar Khot, Varsha Electrtotech, India
A few years ago, Redmond, Wash.’s local fire department received some discouraging news from the county medical director. They were told their service had a recorded CPR compression hands-on ratio of 66 percent—the lowest in the entire county. They knew about the data emphasizing increased compression time to increase resuscitation, so they quickly went to work on a performance improvement plan using new defibrillators they recently purchased from Physio-Control.
After resuscitation, Physio-Control’s LIFEPAK 15 defibrillator would transmit data via high-speed modem through Physio-Control’s LIFENET System to the CODE-STAT database at Redmond Medic One. The system would then send an alert to the smartphones of paramedics designated as “annotators” to let them know they had a case to review. “By the time [responders] got back to the station, they had instant feedback,” says Cam Pollock, global marketing vice president at Physio-Control.
After six months testing out this new feedback system, Redmond Medic One received more news from the county medical director. Their CPR compression hands-on-ratio was now the highest in the country at 83 percent.
“It improved over 20 points in six months, just by feeding back their performance on a quick basis,” says Pollock.
Improving patient outcomes through data
The American Heart Association updates its guidelines for CPR and ECC every five years. The most recent update, from 2010, highlights the need for good data.
“You can’t improve what you can’t measure. If you want to improve patient outcomes, you have to have a good way to collect and analyze,” says Pollock. The LIFENET system is cloud-based and connects to all of Physio-Control's products. Out in the field, not only would the device be able to feed back data to a local station to help paramedics improve their skills, but it could also send data to a local hospital in order for adequate preparations to be made for the patient’s arrival.
Recent AHA guidelines also urged high-quality compressions during CPR, specifically two inches of depth compression and at least 100 compressions per minute. But anyone who has ever performed CPR knows this is hard to maintain.
“Having a device deliver that instruction to the user for CPR is equally as important as the shock in my opinion,” says Ben Wellons, president of eMed Healthcare, a defibrillator distributor based in Little Rock, Ark.
CPR solutions
The top makers of defibrillators all incorporate several pieces of equipment and software that provide direct feedback to users to improve CPR numbers.
“CPR is challenging for people and unless you apply measurement and feedback, the user may not realize the quality,” says Jamie Froman, director of marketing for Philips Healthcare’s HeartStart automated external defibrillators.
Philips’ advanced life support HeartStart defibrillators come with quality CPR measurement and feedback. A little monitor that’s connected to the defibrillator sits on the patient’s chest. It helps measure the depth and rate of the compressions and gives the end user feedback on a display screen so that they can adjust their technique accordingly.
Zoll Medical Corporation, which was recently acquired by Japan’s Asahi Kasei Corp., provides Real CPR Help and real-time audio and visual feedback on all of its defibrillators to coach rescuers to the correct depth and rate of chest compressions as recommended by the AHA Guidelines. A few of Zoll’s defibrillators offer See-Thru CPR, which minimizes interruptions during CPR.
“The user is getting visual feedback on the important elements of quality and they are getting audible feedback when they are out of range,” says Ward Hamilton, senior vice president and vice president of marketing for Zoll. But Hamilton stresses the importance of the kind of feedback received.
“We tried to make sure it’s enough feedback but not too much that it interferes or you tune it out,” he says.
Data management is also a critical part of Philips’ advanced life support HeartStart line. “We can acquire a 12-lead ECG from a patient and send that to a receiving hospital in order for them to determine what hospital they should go to and the appropriate pathway of care,” says Froman. All the data on the device also helps guide the patient’s post treatment of care.
Zoll defibrillators with See-Thru CPR and Real CPR Help affected the University of California San Diego medical team. They tripled survival rates using the devices along with a “resuscitation bundle” as a new paradigm of care — again, using feedback to guide CPR performance and data analysis to aid in team training.
Taking this a step further, Physio-Control’s fastest growing product, the LUCAS 2 chest compression system, is a portable device that’s strapped onto a patient’s chest during cardiac arrest. It delivers automated, consistent chest compressions that aren’t compromised by a professional tiring out.
“Most people don’t do CPR often enough to become experts. In other words, quality declines quickly and rescuers often have to switch off, leaving gaps in the CPR flow. That affects not only overall survival, but also defibrillator efficacy. You want good CPR being done up until the shock. The longer you wait with hands off the chest, the defibrillator shock is less effective,” says Pollock.
Emergency kits
The Zoll defibrillators used by the UC San Diego team also had built in End-Tidal Carbon Dioxide monitoring to guide the quality of the CPR. Users can look for these values, displayed during cardiac arrest, to adequately gauge blood flow and O2 exchange during resuscitation.
Capnography—the measurement and numerical display of End-Tidal Carbon Dioxide—is another important feature highlighted in the 2010 AHA guidelines.
“So if you’re doing CPR and you have a low capnography, then you need to push harder. And there are some recommendations in the guidelines that say if you’re not at a certain level, you should be doing better CPR. It’s really becoming a measure of overall resuscitation,” says Pollack.
The LIFEPACK 15 has capnography measurements built in, but they also sell a standalone capnography module for people who want a hand-held device. Pollack describes this unit as a portable resuscitation unit or emergency care unit because it can do many more things than defibrillation, monitoring and pacing.
Some of the newest parameters on the device are temperature monitoring for hypothermia and a carboxyhemoglobin feature to detect carbon monoxide in the blood and methemoglobin in the blood, which results from exposure to certain drugs and chemicals.
Smaller but just as powerful
This year, manufacturers have released smaller and more compact defibrillators. Zoll’s lightweight, portable X Series Monitor/Defibrillator received 510(k) clearance from the Food and Drug Administration in March.
“We think it’s going to be a game-changing product and will set a whole new standard in that category,” says Hamilton. The device is being marketing to the pre-hospital environment and provides every advanced monitoring and communication capability required by EMS providers. But in a device that’s less than 12 pounds— nearly half the weight of most others on the market.
“Especially when you have to move equipment to a patient’s side, size and weight are key. Sometimes you can get small size, but you have to compromise the screen or features. Here, you have it all and you don’t have to give up anything to get the small size and the light weight,” says Hamilton.
He also points out that the device has two new capabilities: the ability for paramedics to communicate over wireless and to export data onto a USB port.
Phillips claims its new HeartStart FR3 is the smallest and lightest on the market for professional responders at less than four pounds. Some improvements in the device focus on workflow solutions for the users — all based on feedback from EMTs working with Philips’ devices out in the field. One improvement Froman points to is the pre-connected pads. “The engineers have found a way to take the bags off the pad so it removes a step from the workflow when time is of the essence,” he says. The device also has a bilingual capability that Froman thinks will give certain communities access to a wider range of responders.
“As we observe clinicians and ask for feedback to put into our products, over time, some of the features like Q-CPR that we have in the hospital and emergency medical space might migrate into other spaces. We fully expect that to happen,” he says.
DOTmed Registered 2012 - July - DMBN: Defibrillators Companies
Names in boldface are Premium Listings.
Domestic
Katherine MacCall, Soma Technology, CT
Leslie Roberts, Altra Medical, FL
Allyn Cutts, American Med Supply, FL
Moshe Alkalay, Hi Tech Int'l Group, FL
DOTmed Certified
Ronald Tarr, MEDELCO, FL
Todd McCuaig, LS Inc, IL
Alda Clemmey, Saffire Medical, MA
DOTmed Certified
DOTmed 100
John Gladstein, Medical Device Depot, MD
DOTmed Certified
Robert Schirano, Finger Lakes Medical Supply LLC, NY
Lawrence Maroney, Integris Equipment, NY
DOTmed Certified
International
Mads Vittrup, AGITO Medical, Denmark
DOTmed 100
Manohar Khot, Varsha Electrtotech, India